Develop medical device CERs in compliance with MEDDEV 2.7/1 Rev. 4 (European guideline) as well as, reports for other worldwide markets.
Why CERs are More Challenging Now Than Ever Before
Stricter clinical data requirements are now required under the MDD and will continue under the MDR
- CERs are very labor intensive requiring specialized clinical/regulatory experience
- There is increased scrutiny on clinical data
- CERs are required for all devices regardless of risk class and must be updated at least annually
- Planning, collection and analysis of clinical data, and the development of compliant CERs should happen now
CER Process
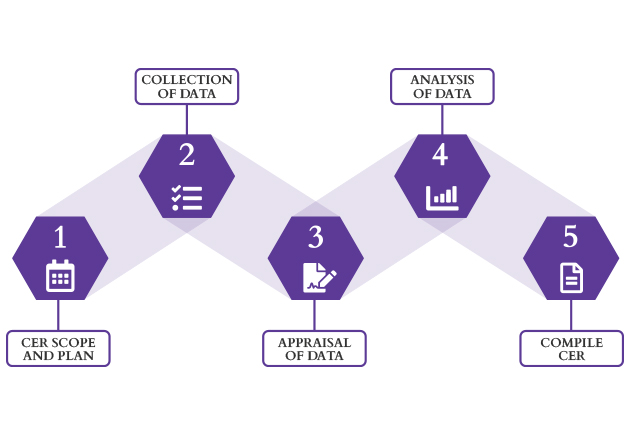
We Assist You Through Every Stage of the CER Process
CER Procedures
Create compliant procedures related to: the development of CERs, conducting literature reviews, and data appraisal methodology
CER Strategic Planning
MCRA can perform an analysis of available data to determine what is required to meet the regulatory level of sufficiency; this includes an assessment of pre-market investigation data and/or equivalent comparator data
CER Drafting
MCRA can develop the entire CER or specific sections per the established Rev 4 guidance including equivalence justifications, clinical investigation summary, literature review (see below), post-market data assessment and summary, and benefit-risk assessment
Comprehensive Literature Review
MCRA can perform comprehensive literature reviews including database searches and safety and performance analyses
Evaluation of Clinical Evidence
As part of the CER process, MCRA will assess all data sources to generate a benefit risk assessment to determine the benefit risk profile of the device; whether any actions are required on the part of your company, and ultimately, support the marketing application of the device
Our Experienced Team
- Years of experience, developing new CERs and updating existing ones
- Team of trained writers with both technical writing and regulatory knowledge
- Capability to conduct a literature review and analysis across multiple databases with multiple reviewers
- Integration of Clinical, Regulatory, & Quality experts who understand the content of a successful CE
- Capacity to act independently on all components with the exception of internal data required from the sponsor
